Science
| Status | Done |
|---|

1.1 – Atoms
Matter is anything that has mass and takes up space. Atoms are small particles made of matter.
Atoms
An atom is the basic building block of all matter. Each atom consists of smaller subatomic particles:
- Protons
- Neutrons
- A cloud of electrons
Models of atoms
Electron Cloud model
Protons and neutrons are found in the nucleus.
Electrons are about 1800x smaller than the other subatomic particles and are much lighter than them.
Electrons exist in an electron cloud that surrounds the nucleus. In this, there are layers of electron shells that contain the actual electrons.
Electrons are unable to naturally leave their designated shell unless they are manually done by humans.
Depending on the element, there are different amounts of shells for each atom – some may have 3 shells and some may have 5.
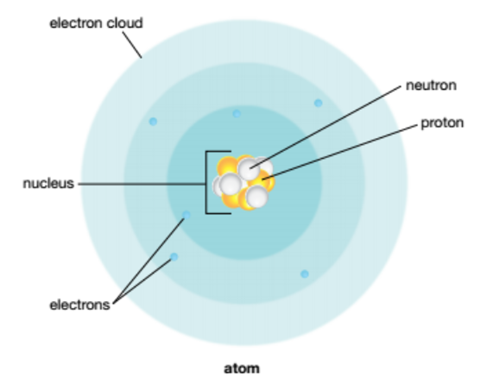
The Bohr model
The Bohr model is not an accurate representation of an atom due to the electrons, however it is still used as it is much easier to draw.
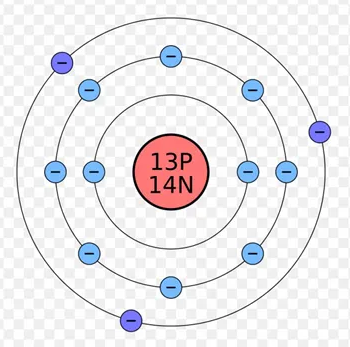
Sub-atomic particles
Protons
Protons have a positive charge (+) and are found in the nucleus of an atom. The number of protons determines the atomic number and therefore what element the atom is.
Neutrons
Neutrons have a neutral charge and are also found in the nucleus of an atom. If the nucleus contains both protons and neutrons, the overall charge of the nucleus will be positive.
The neutrons keep the atom stable and prevent it from radiating and removing a proton/neutron while it is unstable.
Electrons
Electrons are very small, light, and have a negative charge (-). Electrons can be gained or lost in a chemical reaction. All electrons in atoms are found in the electron cloud surrounding the nucleus. They travel randomly in the cloud at discrete energy levels or in shells.
Characteristics of sub-atomic particles
Protons and neutrons are similar in mass; their only difference is their charge.
In an atom, the number of electrons is always the same with the number of protons, thus the negative charge will balance out with the positive charge — so all atoms should be neutral.
Electrostatic attraction and repulsion
Opposite charges attract each other (electrostatic attraction) and the same charges repel each other (electrostatic repulsion).
Why doesn’t the nucleus split due to repulsion?
As the nucleus is positively-charged, the strong force of repelling would be able to split the nucleus.
This doesn’t happen as there is an even stronger force called the nuclear force that keeps the protons in the nucleus together.
The discovery of the atom
Democritus
- Proposed all matter is made of tiny, solid, unbreakable particles called atomos.
- Model: Solid-ball model
John Dalton
- Proposed that combinations of atoms form specific elements or compounds (e.g. H + O + O = water).
- Model: Solid-ball model
J.J. Thompson
- Discovered electrons and their negative charge.
- Proposed atoms are neutral overall, so a positive charge (protons) must exist.
- Model: Plum-pudding model
Ernest Rutherford
- Conducted the gold foil experiment with alpha particles.
- Most particles passed through – atom is mostly empty space.
- Some deflected – came close to nucleus.
- Few bounced back – hit nucleus directly.
- Proposed atoms have a dense, positively charged nucleus with electrons in surrounding space.
- Model: Nuclear model
Niels Bohr
- Modified Rutherford’s model.
- Proposed electrons orbit the nucleus in fixed energy levels.
- Model: Planetary model
Erwin Schrödinger
- Proposed electrons don’t move in fixed paths but in probability-based electron clouds.
- Electrons can be anywhere in a shell; exact location is uncertain.
- Model: Electron cloud model
James Chadwick
- Discovered neutrons in the nucleus alongside protons.
- Neutrons had no charge, making them hard to detect.
- Model: Electron cloud model with neutrons
Calculating the sub-atomic particles
Atomic number
The atomic number is a count of how many protons there are in an atom.
The number of protons in a neutral atom is always equal to the number of electrons.
Mass number
The mass number is a combination of the number of protons and neutrons.
This means if we subtract the atomic number from the mass number, we get the number of neutrons in an atom.
The mass number is usually larger than the atomic number (except in hydrogen, where there are no neutrons).
To calculate the neutrons you do mass number - atomic number.
Notations
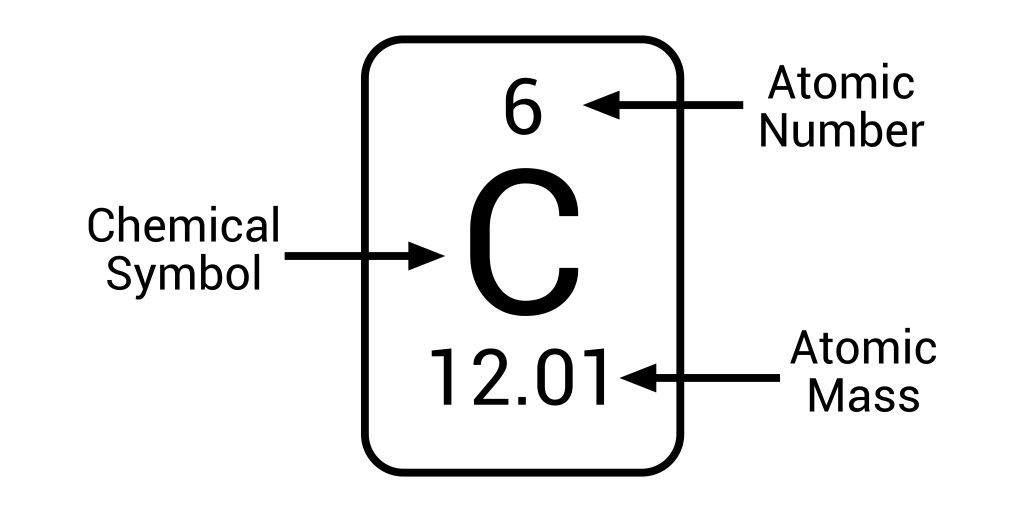
Periodic notation
Periodic notation is how we represent an element on the periodic table. We have to round the atomic mass to the nearest whole number.
Atomic notation
The atomic notation is a bit different to the periodic notation — it has the mass number on top and the atomic number at the bottom, and the numbers are shifted more to the left.
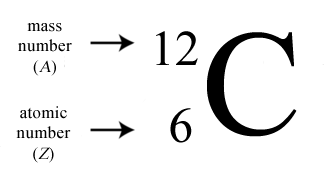
Elements
An element is a pure substance containing only the same type of atoms that have the same number of protons in their atomic nuclei. They are the fundamental materials of which all matter is composed of.
1.2 – The role of electrons and electron shells
Quick terminology
- Molecule: two or more atoms chemically combined, any type of atom.
- Compound: subset of molecules; two or more different atoms chemically combined.
- Mixture: any element or compound not chemically combined; can be separated.
Electron configuration
Electron configuration is the arrangement of electrons in the energy levels around the nucleus.
Each energy level has the maximum amount of electrons that it can hold:
- 1st level can hold 2 electrons.
- 2nd level can hold 8 electrons.
- 3rd level can hold 18 electrons.
- 4th level can hold 32 electrons.
The formula for these energy level-electrons relationship is .
Relationship between periods, groups and electrons
The electrons in the outermost shell or energy level of an atom are called valence electrons.
The number of a group tells us how many valence electrons the element has (for example, Xenon is in the 8th group, and thus has 8 valence electrons).
When moving down a group, the number of valence electrons stay the same, but the number of energy levels increases by one.
When moving across a period, the number of valence electrons increases by one, however the number of energy levels stay the same.
Ions
Ions are formed when electrons are gained or lost from an atom.
If the ion has lost electrons, then the ion is called a cation. If the ion has gained electrons, then the ion is called an anion.
Metals tend to give their electrons away and become cations, while non-metals tend to take electrons and become anions. They do this to achieve a full outer shell. For example, a magnesium atom loses two electrons and so it has a chemical formula of .
Valency
Valency refers to the number of electrons gained, lost or shared in order to achieve a full outer shell. Metals form cations with a valency of +1, +2 or +3 while non-metals form anions with a valency of -1, -2 or -3. This is written as a superscript next to the chemical symbol.
To name cations, we add the word “ion” after the element, like magnesium ion. To name anions, we add the suffix “ide” to the element, like oxide or nitride.
Ionic compounds
Naming
When naming ionic compounds, the metal cation is always first and its name does not change.
The non-metal anion is named second and usually ends in ..ide.
For example:
- Sodium → Sodium
- Chlorine → Chloride
Thus, it is sodium chloride.
Writing the equation: cross-over method
For example:
Take the 2 from the O and put it as the subscript for Na. Take the 1 from the Na and put it on O. Make sure to not write 1 or any of the symbols.
Covalent compounds
A covalent compound forms when two non-metals come together and share electrons — no new ions are formed.
The bond that exists between these two non-metals is called the covalent bond.
Naming
The element farthest to the left is usually named first. If both non-metals are in the same group, then the bottommost element is first.
The second element is named as an anion although they are not ions; they end with the suffix “…ide.”
Prefixes are used to denote how many atoms there are.
Covalent compounds are not ratios and should not be simplified.
| Prefix | Number | Example |
|---|---|---|
| Mono- | 1 | Monoxide |
| Di- | 2 | Dioxide |
| Tri- | 3 | Trioxide |
| Tetra- | 4 | Tetroxide |
| Penta- | 5 | Pentoxide |
| Hexa- | 6 | Hexoxide |
| Hepta- | 7 | Heptoxide |
| Octa- | 8 | Octoxide |
| Nona- | 9 | Nonoxide |
| Deca- | 10 | Decoxide |
Oxygen/halogen exception
If a covalent compound contains a oxygen and a halogen (Chlorine, Bromine or Iodine), the halogen will always be written first. The only halogen that does not do this is fluorine, which is named second.
For example, OF2 is oxygen difluoride.
Metals and non-metals
Metals are pure, solid substances with particular qualities.
A non-metal is a substance that does not have the quantities of metals.
| Property | Metals | Non-metals |
|---|---|---|
| Lustre | Lustrous | No lustre |
| Hardness | Very hard | Not hard |
| Malleable (hammered into sheets) | Malleable | Brittle |
| Ductile (stretched into wires) | Ductile | Non-ductile |
| Conductivity | Good conductors of heat and electricity | Poor conductors of heat/electricity |
| State | Solid (except mercury as a liquid) | Solid, liquid or gas (only bromine is liquid) |
| Density | Very dense | Not dense |
Uses and properties of metals
Metals are used based on the properties they have.
| Pure metal | Uses | Property that make it particularly suited to its use |
|---|---|---|
| Copper (Cu) | Electrical wiring, water pipers | Excellent electrical conductor, very ductile |
| Tin (Sn) | Coating for steel cans used for storing food | Stops steel from rusting, doesn’t react with food, non-toxic. |
Alloys
An alloy is a mixture of a metal with small amounts of other elements.
The properties of an alloy are usually an improvement over the original base metal.
For example, the alloy steel is much stronger and durable than its base metal iron.
Uses of some alloys
Stainless steel is made by adding carbon and chromium to iron, making it rust resistant and used in kitchens, ships, surgical instruments and jewellery.
Brass is composed of 70% copper and 30% zinc. It is used for hinges, door handles, fittings on boats and ships and musical instruments. It is good looking, doesn’t corrode much, and is stronger than its base metal copper.
Cupronickel is 75% copper and 25% nickel. It is used for ‘silver’ coins. It is hard wearing and looks like silver.
Metalloids
Metalloids or semi-metals show properties of both metals and non-metals.
Silicon and germanium are used in electronic components because they are semiconductors. This means that under certain conditions, they can conduct electricity which makes them similar to metals.
However, they are also similar to non-metals because they are brittle and are poor conductors of heat.
1.3 – pH, acids and bases
pH scale
pH is a measure of ion concentration, which tells us how acidic a solution is.
The pH scale ranges from 0-14.
Acidic solutions have pH values below 7 — a solution with a pH of 0 is very acidic. A solution with a pH of 7 is neutral (water is pH 7), and basic solutions have pH values over 7.
If one solution has a pH of 1 and a second solution has a pH of 2, the first solution is 10 times more acidic than the second solution.
Acids
An acid is an substance which is capable of releasing ions into solution. The more ions released, the more acidic the solution.
Properties of acids
Acids:
- Are corrosive - can cause burns
- Have a sour taste - like vinegar
- Turn blue litmus paper red
- React with some metals, releasing hydrogen gas and leaving a salt behind.
- Conduct electricity
- Neutralised by bases - producing water and a salt.
Acid strength
The strength of an acid depends on how many ions are released into solution by an acid. It refers to how likely it is to release ions into solution.
If all of its ions are released into solution, then it is a strong acid. If only some of the ions are released into solution, then it is a weak acid.
Acid concentration
Concentration refers to how much acid is present in a solution.
A concentrated acid has a high amount of acid molecules in the solution. A dilute acid has a little amount of acid molecules in the solution.
There are four types of these: concentrated weak, concentrated strong, dilute weak, and dilute strong.
Examples of strong and weak acids
Sulfuric is a strong acid that has a chemical formula of H2SO4 and is used in making other chemicals, dyes, fertilisers, etc.
Ascorbic is a weak acid that has a chemical formula of C6H3O6 and is found in Vitamin C.
Carbonic is a weak acid that has a chemical formula of H2CO3 and is found in rain water and fizzy soft drinks.
Acetic (ethanoic) is a weak acid that has a chemical formula of CH3COOH and is found in Vinegar.
Measuring pH using indicators
Indicators are chemicals that change colour to show whether a substance is acidic, neutral or basic.
Litmus paper turns red in acid and blue in bases, but it does not tell the pH. Other indicators like universal indicator does.
Bases
A base is a substance that is capable of releasing hydroxide ions into solution.
Not all bases can dissolve in water. If it does, it is called an alkali solution.
Properties of bases
Bases:
- Are caustic - capable of burning or corroding.
- Have a soapy, slimy feel.
- Turn red litmus paper blue.
- Have a bitter taste.
- Conduct electricity.
- Neutralised by acids producing water and a salt.
Base strength
A strong base releases all of its ions into solution. A weak base releases only a few of its hydroxide ions in solution.
Examples of strong and weak bases/alkalis
Sodium hydroxide (caustic soda) is a strong base has a chemical formula of NaOH and is used for producing soap, stripping paint, and to clean drains and ovens.
Ammonia is a weak base has a chemical formula of NH3 and is found in household cleaners. Ammonium hydroxide has a chemical formula of NH4OH and is also found in household cleaners.
1.4 — New materials
New materials are created for social, ethical and environmental needs.
Polymers and synthetic fibres
Polymers are molecules made of repeating units called monomers. Most polymers are synthetic, such as plastic.
Fibres are substances that can be twisted, woven or knitted — their molecules are tangled, and not always chemically joined.
Self-cleaning fabrics
Self-cleaning fabrics are coated in molecules of polymers making the surface hydrophobic (water repellent; opposite is hydrophilic).
Water drops are nearly spherical when they land on hydrophobic surfaces, in an attempt to avoid contact with the surface. The angle the droplet lands on is called the contact angle.
Carbon fibres
A carbon nanotube is a tiny cylinder of carbon atoms that is 90-100 nm long. The properties of carbon nanotubes changes depending on what angle you roll the sheet.
The fibre can be much stronger than steel and much lighter.
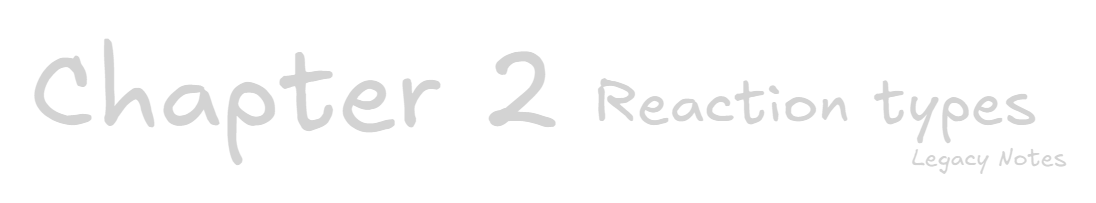
2.1 – Combustion and corrosion reactants
Signs of a chemical reaction
- Change in colour
- Change in temperature/production of light (energy/heat is produced or absorbed)
- Formation of a precipitate when two clear solutions are mixed
- Formation of a gas/smell or seeing bubbles form.
Reactants and products
In a chemical reaction, the starting substances are called the reactants, and the new substances formed are called products. It is difficult to turn products back into reactants.
The reactants are on the left of the arrow and the products are on the right.
Endothermic and exothermic reactions
Endothermic and exothermic reactions refer to the absorption or release of heat.
In an exothermic reaction, heat is released from the system into the surroundings. Thus, the temperature of the surroundings increases.
In an endothermic reaction, heat is absorbed from the surroundings into the system. Thus, the temperature of the surroundings decreases. When measuring a substance with a thermometer, the thermometer is a surrounding object.
Endothermic reactions
These reactions need energy to proceed, so they get the energy from their surroundings as heat.
An example is an ice pack applied during an injury. Packets of ammonium nitrate and water are broken, allowing these substances to mix and react. As they do so, they absorb energy from their surroundings (the packet/your skin), cooling the surroundings down.
Exothermic reactions
These reactions release energy into the surroundings in the form of heat or light.
Some examples include combustion (burning), respiration (breathing), and when liquid water turns into ice (the liquid water releases energy turning into ice).
Combustion
Combustion reactants are exothermic reactions, where light and heat are released. It occurs when a substance reacts with oxygen gas. The main products of this are carbon dioxide and water.
Petrol is a mixture of hydrocarbons, the most important one being octane.
When there is a plentiful supply of oxygen available, complete combustion occurs resulting into the production of heat, carbon dioxide and water.
If there is a lack of oxygen available, incomplete combustion occurs resulting in the production of less heat, carbon monoxide gas, water and sometimes carbon soot.
Complete: methane + oxygen gas → carbon dioxide + water vapour
Complete word equation: + → + + heat
Incomplete: methane + oxygen gas → carbon monoxide + water vapour
Very Incomplete: methane + oxygen gas → carbon + water vapour
Aerobic cellular respiration
A much slower and controlled example of combustion is aerobic respiration, when glucose and oxygen combine to form carbon dioxide, water and energy called ATP.
glucose + oxygen gas → carbon dioxide + water
+ → +
Magnesium combustion
Not all combustion reactants produce carbon dioxide and water vapour. For example, when burnt, magnesium reacts with oxygen to form magnesium oxide.
magnesium + oxygen gas → magnesium oxide
Corrosion
Most metals corrode when exposed to water, air or other chemicals. Corrosion is a chemical reaction that form other compounds from these metals.
Iron/steel bodies of cars will react with oxygen and water until all that is left is a pile of rust.
Corrosion of copper
Copper corrodes in a similar way but instead of producing an orange residue it produces a green one called verdigris - a combination of copper hydroxide and copper carbonate.
copper + water + carbon dioxide + water vapour → copper (II) hydroxide + copper (II) carbonate
Corrosion of silver
Pure silver reacts with sulfur to form a black coat called tarnish (silver sulfide). The sulfur comes from hydrogen sulfide, which is found in air pollution or from foods such as eggs, fish, onion and pea soup.
silver + hydrogen sulfide → silver sulfide + hydrogen gas
Rusting of iron
When iron and some poor grades of steel are exposed to oxygen and water, they form rust or iron oxide.
Rust is flakey and easy to dislodge allowing the rusting process to continue further into the next layer of the metal, causing the metal to become weaker and thinner.
iron + oxygen gas → iron (III) oxide
Corrosion of aluminium
Aluminium is a reactive metal. Its surface reacts with oxygen in the air to form a fine layer called aluminium oxide.
aluminium + oxygen gas → aluminium oxide
Unlike rust, this layer does not flake but instead acts like a layer/barrier that protects the aluminium metal below.
Anodising is a process that deliberately creates an oxide layer on metals to protect them.
Antoine Lavoisier’s theory
As rusted metals were thought to have lost mass due to the phlogiston theory, it was absurd that it had gained mass instead.
Mass cannot be created, therefore there was a missing element in the equation - oxygen.
2.2 – Acid reactions
Acids and metals
When acids and metals react with each other, the general equation is:
Acid + metal → Hydrogen gas + salt
The term ‘salt’ refers to any compound formed by a metal (cation) taking the place of the hydrogen in an acid.
Acids and bases (neutralisation)
When an acid and base are mixed, they neutralise each other producing a salt and water:
Acid + base → Salt + water
Acids and carbonates
When an acid and carbonate are mixed, they produce a salt, water and carbon dioxide gas.
Acid + carbonate → Salt + water + carbon dioxide
To prove that carbon dioxide is a product of an acid + carbonate reaction, the gas can be bubbled into a test tube of limewater. This will produce a precipitate of , turning the solution milky.
Acid reactions in digestion
Chemical digestion is the process that breaks down large molecules in food into smaller ones that can be absorbed by the body.
Enzymes are ‘helper chemicals’ that assist in speeding up digestion.
Types of enzymes
Different types of enzymes need different environments to survive in.
For example, amylase is found in saliva and helps digest starch. It can only survive when the pH of the mouth is around 7.
Pepsin in your stomach helps digest proteins. It can only survive and function in the stomach where the environment is acidic with a low pH.
The stomach is too acidic for amylase so digestion of starch stops in the stomach, and the digestion of protein begins.
As the digested material exists the stomach and enters the small intestine (duodenum), it is far too acidic to pass through the rest of the digestive tract.
If the pH of the duodenum falls below 5, the pancreas releases sodium hydrogen carbonate to neutralise the acid left in the digested material.
This returns the pH of the duodenum to 7 or 8 (ideal pH for pancreatic enzymes), which now allows the enzymes of the pancreas to digest the remaining starch, proteins and fat.
2.3 – Reactions of life
Photosynthesis
Photosynthesis is a series of chemical reactions that green plants use to product the sugar glucose,
Photosynthesis can take place in any part of the plant, but mostly occurs in the leaves, because they are the most exposed parts.
Obtaining the raw materials for photosynthesis
The reactants of photosynthesis are carbon dioxide and water. Chlorophyll and sunlight are also required in order for this to work.
- Water is carried from the roots up the plant’s transport system called the xylem, and into the leaves.
- Chlorophyll is found in the chloroplasts within the leaves. These chemicals help convert light energy into chemical energy that can be used. The chloroplasts also contain enzymes which helps speed up photosynthesis.
- Carbon dioxide moves from the air into tiny openings in the left called stomata (operated by guard cells) by the process of diffusion — gas from higher concentrated areas move to the lower concentrated areas, and therefore into the stomata. The carbon dioxide diffuses from the air into the lower parts of the leaf, then makes its way up to the chloroplasts where it is used to carry out photosynthesis.
Aerobic respiration
Respiration is the process through which plant and animal cells release energy (ATP) from glucose.
Plants get glucose by carrying out photosynthesis and animals get it through the digestion of food. Both use a form of respiration called - aerobic respiration.
Aerobic respiration needs a supply of oxygen as well as glucose. The oxygen is circulated in our bloodstream combining with glucose to form carbon dioxide, water and energy.
Respiration in plants
Oxygen is brought to the cells of a plant through specialised structures called lenticels, found in the stems and roots.
Carbon dioxide diffuses out of a plant in several ways. Most are taken in by the chloroplasts to be used in photosynthesis, and some are taken out of the mitochondria, into the cytoplasm and leaves the cells, exists through the stomata back into the atmosphere.
Water diffuses and evaporates out from the cell through the stomata.
Carbon sinks
Plants release carbon dioxide back into the atmosphere, but in smaller quantities than that used up by photosynthesis. This is because glucose isn’t used only for plant respiration, but also to build the plant itself.
Glucose is often:
- Converted into starch for short- or long-term storage
- Converted into cellulose to build cell walls
- Converted into substances to make fats (olive oil) or proteins.
- Converted into sucrose and transported to parts of the cell where photosynthesis cannot occur (roots)
- Used to make vitamins.
By doing all this, plants remove carbon dioxide from the atmosphere via photosynthesis and store it. This is why they are called carbon sinks.
When trees and forests are chopped down, they cannot take in any more carbon. When they are burnt, the stored carbon is released back into the atmosphere as carbon dioxide.
Respiration of humans
The blood stream of animals carries glucose and oxygen to the cells of the body. Glucose is received through digestion of food, oxygen via breathing.
Oxygen passes through structures in the lungs called alveoli and then diffuses into the blood.
Removal of waste
The blood also carries away the waste products of respiration which are carbon dioxide and water.
Some water is reabsorbed into the body, some is removed by the kidneys as urine, through sweat, and some is also breathed out.
Carbon dioxide diffuses out from the blood into the alveoli in the lungs, then is also breathed out.
Comparing photosynthesis and respiration
Respiration and photosynthesis are exact opposites of each other. Photosynthesis makes glucose and oxygen, respiration uses them.
Even though both photosynthesis and respiration require some ATP (energy), respiration produces a lot more ATP that in uses up. Photosynthesis does not make any ATP.
2.4 – Nuclear reactions
Nuclear/radioactive decay
Combustion, corrosion, neutralisation, etc are all types of chemical reactions that convert the reactants into products — the atoms just rearrange to form the products. It also involves the transfer/sharing of electrons.
To convert an atom into a different atom, it requires a change in the nucleus. This is known as a nuclear reaction.
Nuclear decay is the process by which an unstable atom emits radiation in order to become stable. This results in a new element being formed.
For example, a sodium atom will undergo nuclear decay by losing a proton, and will turn into a Neon atom. This process of an atom converting into a different type of atom is known as transmutation.
Isotopes
Isotopes are different ‘versions’ of the same type of element. ₁¹H, ₁²H, and ₁³H are all isotopes of hydrogen.
They have the same number of protons but a different number of neutrons. So, they have the same atomic number but different mass numbers.
If the isotope has an unstable nucleus, it will undergo nuclear decay to become stable. These isotopes are called radioisotopes. If the isotope has a stable nucleus, it will not undergo nuclear decay.
What makes a nucleus unstable?
A nucleus is stable when the number of protons and neutrons in the nucleus is in a balanced ratio. If there are too many protons/neutrons the nucleus becomes unstable.
Once it is unstable it must undergo radioactive decay and emit radiation, to become stable again.
Isotopes of carbon
Carbon has 3 naturally occurring isotopes: carbon-12, carbon-13 and carbon-14.
- Carbon-12 has 6 protons and 6 neutrons.
- Carbon-13 has 6 protons and 7 neutrons.
- Carbon-14 has 6 protons and 8 neutrons.
Carbon-14 is a radioisotope because it has an unstable nucleus. Therefore it will undergo radioactive decay and turn into a different, more stable atom.
Geiger counter
A Geiger counter is a tool that is used to detect the presence of radioisotopes.
Types of nuclear decay
There are 3 types of nuclear decay that radioactive isotopes will undergo in order to become stable.
Alpha decay
If the number of protons in the nucleus of an atom is too high, it will undergo alpha decay to change into a different, more stable atom.
Alpha decay emits an alpha particle which consists of 2 protons and 2 neutrons. The particle is identical to a helium nucleus, ⁴₂He² ⁺, and it carries a +2 charge.
Therefore the alpha particle is given the symbol α or ⁴₂He² ⁺.
Beta decay
If the number of neutrons in the nucleus of an atom is too high, it will undergo beta decay to change into a different, more stable atom.
Beta decay converts a neutron into a proton and ejects a beta particle, ⁰₋ ₁e, which is very similar to an election (small and negatively charged).
This increases the atomic number by 1, meaning the new element is formed, but the mass number remains the same.
Carbon-14 has 6 protons and 8 neutrons. It undergoes beta decay, ejecting a beta particle (electron) and converting 1 neutron into a proton, forming nitrogen-14 with 7 protons and 7 neutrons.
Gamma decay
Sometimes the protons and neutrons simply rearrange inside the nucleus, but do not emit a particle. Instead they emit a gamma ray. This process is known as gamma decay.
Gamma rays are given the symbol γ.
Gamma rays usually accompany alpha and beta decay. They are more powerful than X-rays.
Half life
The half life of a radioisotope is the time it takes for half of the nucleus to decay OR the time it takes for half of the radioisotope to remain.
For example, the radioisotope radon-222 decays in polonium-218 with a half life of 4 days. This means that every 4 days half the remaining amount of Radon-222 will decay.
Every radioisotope has a different half-life, varying from seconds to millions of years.
To find the value of a half life, you use the formula: .
Carbon dating
Carbon-14 is used to date the age of fossils. This is called carbon dating.
When an organism dies, carbon is no longer absorbed. At that point, the small amounts of carbon-14 begins to decay into nitrogen-14 with a half life of 5730 years.
If an animal died with 1000 carbon-14 atoms, then after 5730 years there would be 500 carbon-14 atoms and 500 nitrogen-14 atoms.
After a second half life, there would be 250 carbon-14 and 750 nitrogen-14.
After a third half life, there would be 125 carbon-14 and 875 nitrogen-14.
By 50 thousand years, there will be too little carbon-14 left to detect, that it will no longer be an accurate way to determine the age of fossils.
Biological effects of radiation
Alpha, beta and gamma particles can cause damage to cells as they all emit ionising radiation. This means they can remove electrons from atoms and molecules in the body, turning them into ions.
This causes the cells to die or become mutated.
Effects of cell death
Cells that have died from ionising radiation may result in radiation burns or sickness.
- Radiation burns are caused from short exposure to large amounts of radiation. It damages skin cells and organs, causing inflammation and blistering.
- Radiation sicknesses are caused by long exposure to low amount of radiation. Symptoms of this will not appear immediately.
Effects of cell mutation
Cell mutation occurs when ionising radiation damages DNA inside cells. The cell is now damaged and cannot function properly, resulting in the development of a cancer.
The longer you are exposed to ionising radiation, there is a greater chance of cell mutation, therefore a greater chance of cancer.
Mutation in sperm or egg cells can result in genetic diseases affecting the offspring.
Radiation
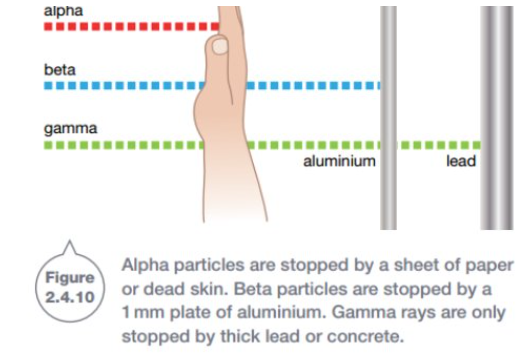
Alpha radiation
Alpha particles are large, heavy and slow compared to beta particles and gamma rays, making them 20x more powerful/ionising than beta particles.
However, they cannot travel far and are easily blocked by a thin sheet of paper or dead layer of skin. Therefore, radioisotopes that release alpha radiation can be handled relatively easily.
However if the alpha radiation enters the body, the effects can be fatal. For example, radioactive gases that emit alpha radiation are very dangerous to the lungs.
Beta radiation
Beta particles are small and fast, allowing them to penetrate the skin more deeply than alpha particles. This is why beta radiation is more likely to cause radiation burns than alpha decay.
Beta radiation can be blocked by a thin sheet of metal/aluminium.
Gamma rays
Gamma radiation can travel through skin, bone and aluminium, making it extremely dangerous to humans. Only a thick layer of concrete or lead can block the radiation.
Gamma rays are a form of electromagnetic ration and not made from particles like in alpha and beta radiation. This means gamma rays do not have any mass, charge and travel at the speed of light.
Applications of radiation
Medical applications
Radiotherapy is a treatment that uses radiation to medically treat cancers. It is when a cancerous tumour is exposed to high concentrations of radiation, to kill the cells in the tumour and stop them from multiplying.
However, healthy cells may also be damaged, and as a result, radiotherapy comes with serious side effects.
Radioisotopes can also be used for medical diagnosis. They can be used to obtain detailed images of organs inside the body — this is called nuclear imaging.
To obtain an image of the internal organs, radioisotopes are injected into the body, and they collect in the organs and emit a very low dose of gamma radiation.
Industrial applications
Radiation is commonly used in the process of sterilisation to kill bacteria in medical equipment and food, so harmful chemicals will not be necessary. It also increases the lifespan of food before it rots.
Radiation can also be used to ‘look’ inside object the same way an X-ray does. A similar technique is used to measure thickness.
Americium-241 is used in smoke detectors. It rings when alpha particles are blocked — this happens when smoke is present.
3.1 — Heat
The law of conservation of energy
The law of conservation of energy states that energy cannot be created nor destroyed, it can only be transformed into different forms.
The particle model
The particle model states that all matter is made up of particles which are in constant motion.
- In a solid, particles are closely packed and can’t move much.
- In a liquid, the particles are closely packed, but are free to move or flow over each other.
- In a gas, the particles are not bound together and are free to move around its container in straight lines until it hits a wall or another particle.
When heat is added to a substance, it increases the kinetic energy of the particles in the substance, making the particles vibrate and move faster, eventually detaching from their bonds and spreading apart.
Heat and temperature
Temperature is a measure of the average kinetic energy of the particles in a substance. The temperature is a value for only one particle in a substance, because it is an average. The more kinetic energy, the higher the temperature.
Heat is the total energy of all the particles in a substance. This is a total, so this is for all particles in a substance. It is determined by the:
- Temperature of the particles (their average kinetic energy)
- Number of particles (mass)
Heat is transferred from a warmer object to a colder object.
Absolute zero
Absolute zero is the point in temperature at which the particles stop moving (no kinetic energy). is this point — it can also be expressed as .
.png)
Thermometers
The element inside a thermometer is either mercury or ethanol — these substances are liquid at room temperature. When heat is applied to the thermometer, the liquid expands due to the particles gaining more kinetic energy and moving more quickly.
When heat is withdrawn from the thermometer, the liquid contracts due to the particles losing kinetic energy, which are now moving much slowly. Eventually, the temperature reaches absolute zero, and the particles now have no more kinetic energy, coming to a complete stop.
When temperature is increased, volume increases and density decreases. When temperature decreases, volume decreases and density increases.
Changing states of matter
When sufficient heat energy is added the particles begin to vibrate and move rapidly until they break free from their bonds that connect each other and the substance changes state.

Heat transfer
Heat flows from areas of higher temperature to those of lower temperature. The greater this temperature difference, the faster the heat flows from one object to another.
Heat transfer can occur in 3 ways: conduction, convection and radiation.
Conduction
Conduction occurs when two objects come in contact with one another and transfer heat. The hotter object transfers heat to the cooler object.
The vibration/energy is passed on when the particles come into contact with each other.
For example, if your hands are hot and you shake your friend’s hand, the heat from your hand is transferred to your friend’s hand.
If you touch a stovetop, the heat from the stove transfers into your hand, making your hand hot. The stove (assuming that it’s on and on heat) will quickly regain the heat it lost from your hand, so it doesn’t become cold.
Conductors of heat
Substances that transfer heat quickly are known as conductors.
Different materials vary on their conductivity. For example, a glass cup is a better conductor than a polystyrene cup.
In other words, the heat from tea flows much better in a glass cup than in a polystyrene cup, therefore glass is a good conductor and polystyrene is a poor conductor of heat.
Insulators
Insulators are substances that are good at trapping heat. An example of these are gases, woollen jumpers, etc.
Woollen jumpers and blankets trap air, in order to insulate your body and keep you warm. They do not allow heat to escape into the air.
Polar bears and penguins have adaptations that reply on insulation to keep them warm.
Convection
As air is heated, its particles gain energy and move further apart. The hot air is less dense than cool air, so it rises, because it is being pushed by the cooler air around it. This method of heat transfer is called convection.
The airflow current is called the convection current.
Heat is transferred by convection in liquids and gases only because their particles can move around, unlike solids where they remain fixed in position.
Radiation
Radiation transmits heat as invisible waves that travel at the speed of light . They are in the form of infrared rays. An example of this is fire radiating heat energy.
The sun cannot transfer heat energy through conduction or convection as these methods cannot travel through empty space. This is why the sun transfers its heat energy through radiation of infrared waves.
Heat transferred by radiation can be easily blocked. For example, sitting in the shade will block the infrared waves of the sun.
The hotter something is, the more heat it radiates.
When radiated energy hits a surface, it can either be absorbed, reflected or passed through the surface. The colour of the surface will often impact this.
3.2 — Sound
Sound is a type of energy made by vibrations. It is produced when something vibrates, moving back and forth very quickly.
These vibrations create sound waves that move through mediums: these include air, water and wood.
When an object vibrates, it causes movement in the particles of the medium, producing a sound wave. This sound wave causes your ear drums to vibrate, allowing you to hear different sounds.
Types of waves
There are two types of waves — transverse and longitudinal. A wave carries energy from one point to another, which can happen in these 2 ways.
For example, the energy carried by waves at a beach move horizontally, but the water particles making up the waves actually move in a vertical direction. This is an example of a transverse wave.
This explains why a boat in the ocean moves up and down as the wave travels across to the shore.
Longitudinal waves
Sound waves are longitudinal waves, which means the particles that make up the waves move back and forth in the same direction as the energy the wave is travelling.
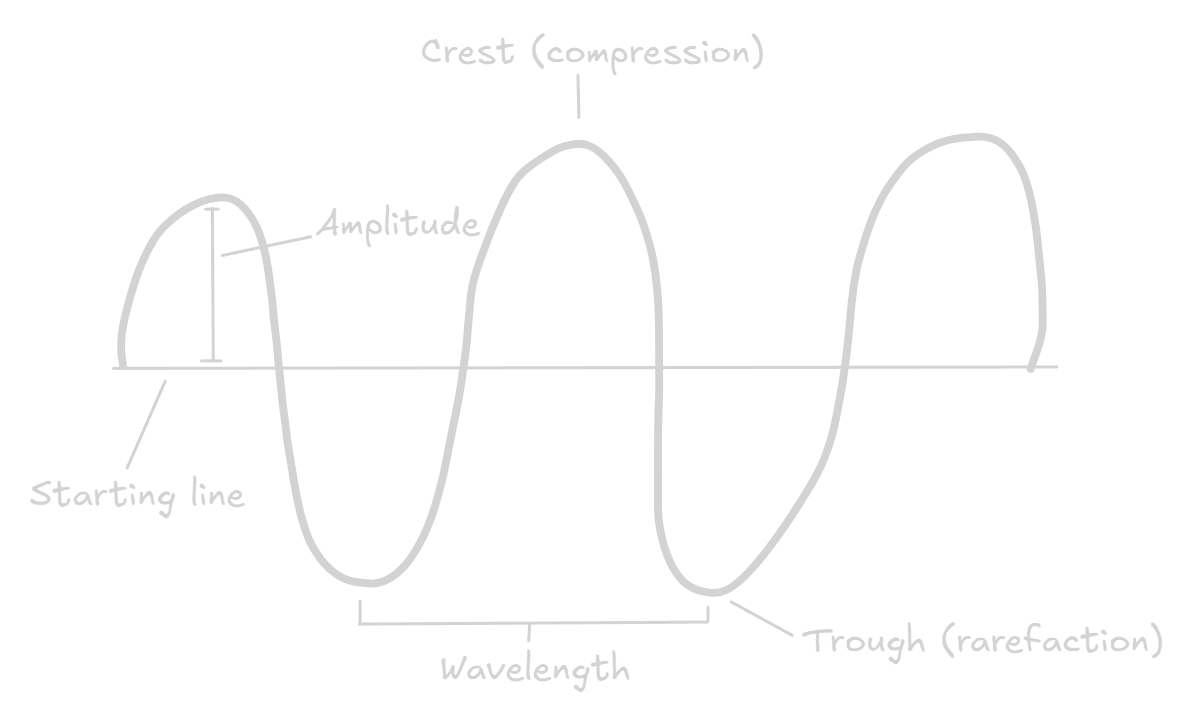
Longitudinal waves have 2 distinct regions.
Compressions are a region where the particles are the closest together, whereas rarefactions are the region where the particles are furthest apart.
Features of a longitudinal wave
The wavelength is the distance from one peak of a wave to the next, from a compression to the next, or from a rarefaction to the next.
The frequency is the number of waves passing a point every second. It is measured in hertz (Hz).
Amplitude is the maximum displacement from a position of rest. It is a measure of the loudness of sound — the larger the amplitude, the louder the sound.
A period is the time taken by the wave to move one wavelength.
Wavelength and frequency are inversely proportional, meaning when one increases, the other decreases. The higher the frequency, the higher the pitch.
Longitudinal waves drawn as transverse
Longitudinal waves are drawn as transverse waves because it is easier to show and label the different features of the wave. It is important to understand that the particles in a soundwave are longitudinal in nature and move in the same direction (parallel) as the energy in that wave.
Speed of sound
The speed of sound depends on the type of medium and the temperature of the medium.
The more closely packed the particles are in the material, the faster the series of compressions and rarefactions will travel. Therefore, sound travels faster through solids than liquids and gases.
The particles of warmer materials vibrates faster than particles in cooler materials, so sound travels faster through warm air than through cool air.
3.3 — Light
Light is a form of energy called electromagnetic radiation.
It travels as a wave, called an electromagnetic wave. All electromagnetic waves are transverse waves. They have the ability to travel through a vacuum, like from the Sun, where the light travels through empty space to reach Earth.
Light travels at the speed of in a straight line.
Transverse wave as light
In a transverse wave, the particle movement is perpendicular (up and down) to the direction of the wave.
In the case of light:
- Amplitude is the intensity or brightness of the light.
- Wavelength is the distance between the two successive crests or troughs of the light wave.
- Frequency is the number of cycles of light that pass a given point in one second.
The wavelength and frequency of a light wave determines its colour and energy.
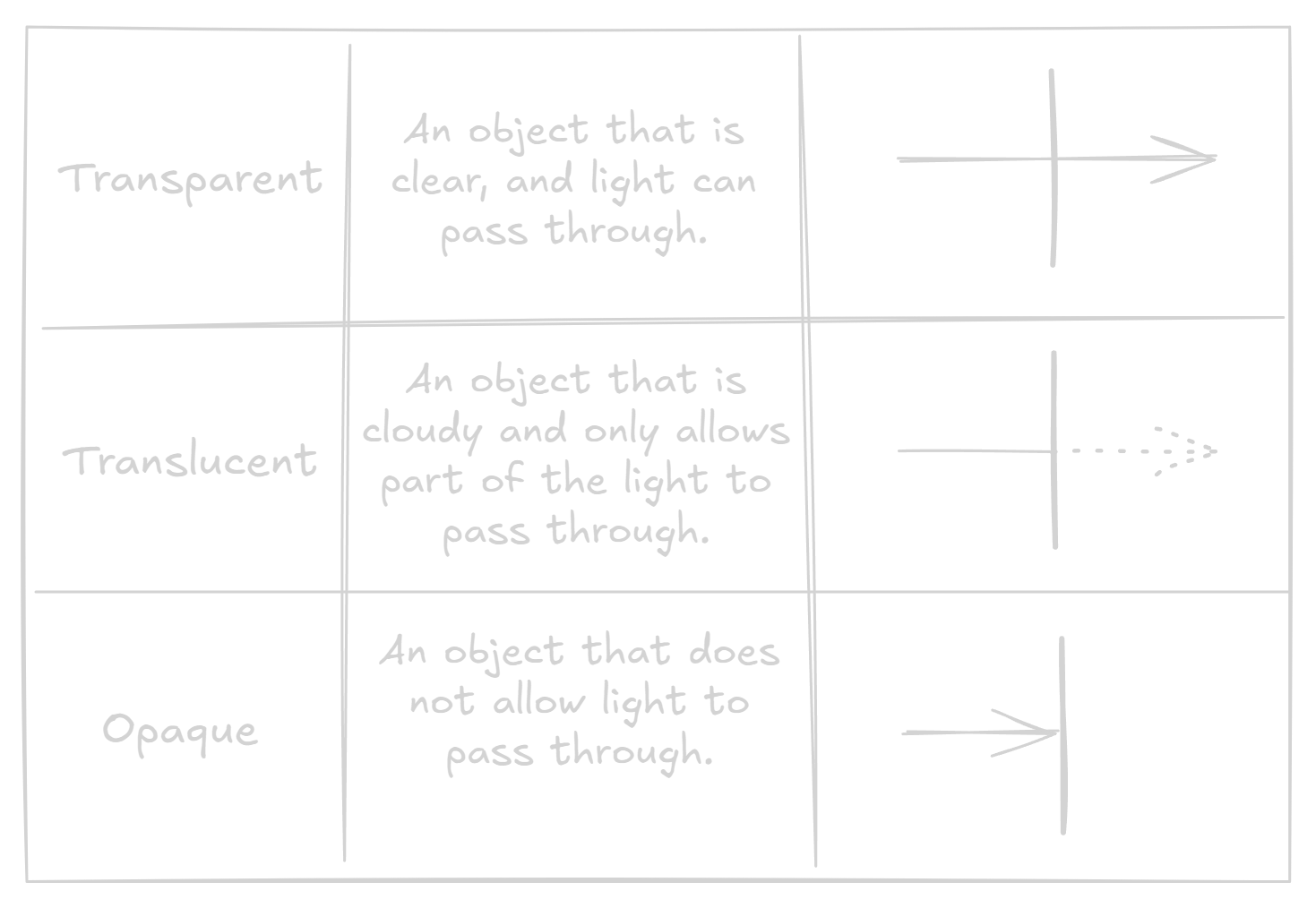
Transparent, translucent and opaque objects
Luminous and non-luminous objects
Luminous objects generate or emit their own light, like the sun.
Illuminated or non-luminous objects are capable of reflecting light into our eyes, for example the moon.
Colour
In 1666, Newton passed a narrow beam of light through a glass prism, and as light exited the prism, he could see the colours of the rainbow. This is called the dispersion of light — when white light is separated into its component colours.
Newton realised that white light consisted of all the colours in the visible spectrum. He classified these colours as red, orange, yellow, green, blue, indigo and violet.
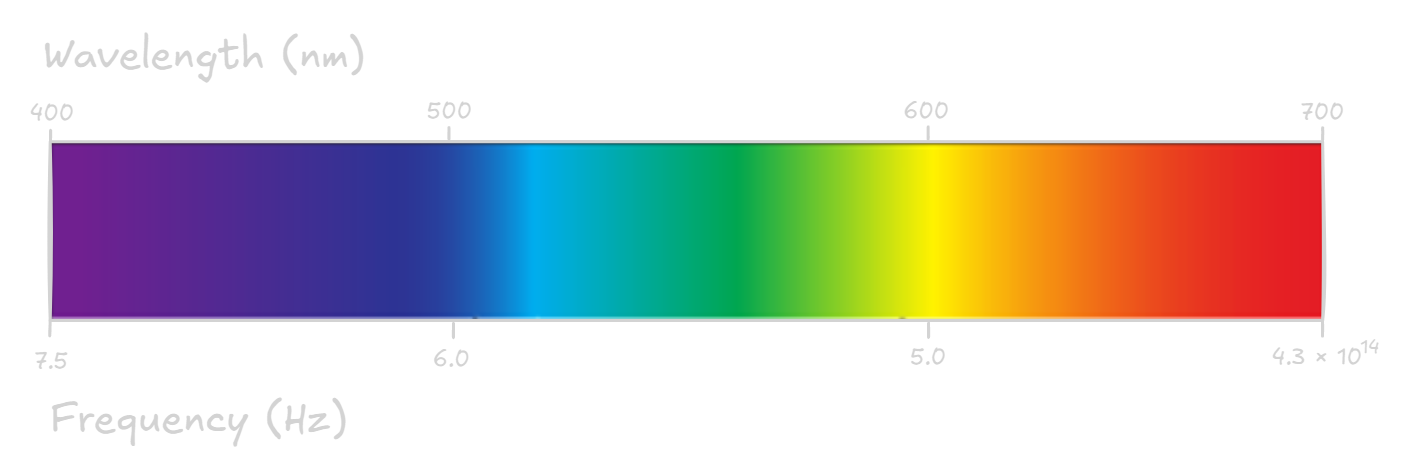
Each colour of light has a different wavelength and frequency. The wavelengths of visible light are extremely small, ranging from violet light (wavelengths of around 400nm), through to red light (around 700nm).
High and low energy electromagnetic radiation
Electromagnetic radiation can be classified as either low or high energy — visible light lies between the two.
Low energy EMR includes radio-waves, microwaves and infrared waves, while high energy EMR includes ultraviolet rays, x-rays, gamma radiation (these are ionising).
In order from lowest to highest frequency (least deadly to more deadly):
- Radio waves
- Microwaves
- Infrared rays
- Visible light
- Ultraviolet rays (UV)
- X-rays
- Gamma rays
Seeing colour
The colour an object appears depends on the colours of light it absorbs and reflects. For example, a red book absorbs all colours, but only reflects red light, therefore it appears red.
Using coloured light
If we looked at a coloured object in coloured light, we see something different.
- If we see a football kit in white light, the shirt looks red and the shorts look blue.
- In red light, the shirt looks red while the shorts look black.
- In blue light, the shirt looks black but the short looks blue.
A pair of purple trousers would reflect purple light, as well as red and blue (due to purple being made up of red and blue).
A white hat would reflect all seven colours.
Using filters
Filters can be used to ‘block’ out different colours of light. They absorb all colours and only allow the light of the same colour as the filter to pass.
When blue light from a blue filter hits a blue object, it will reflect and appear blue, however when it hits a red object, the blue will be absorbed and no light will be reflected, giving the object an appearance of being black.
Reflection
Reflection is the bouncing back of a light ray after it has hit a surface.
The incoming light way is called the incident ray, while the reflected light is called the outgoing ray or reflected ray.
The law of reflection states that when a ray of light is reflected off a surface, either smooth or rough, the angle of incidence is always equal to the angle of reflection.
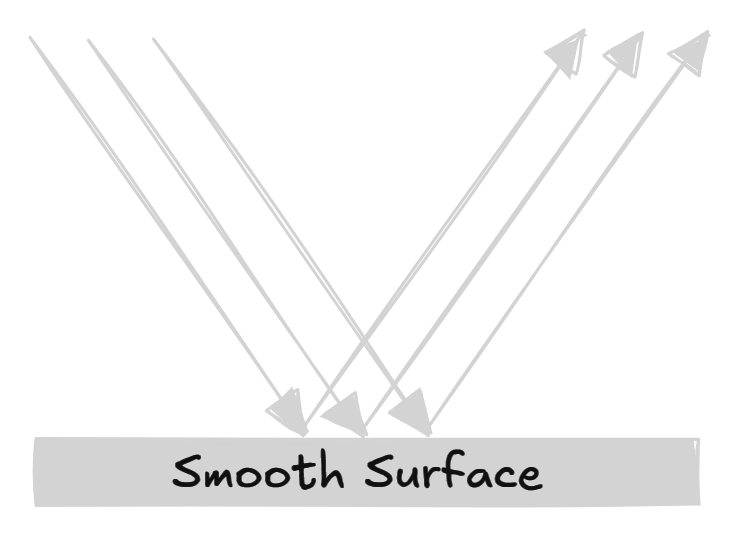
Regular reflections
When light rays fall on a smooth surface, all the light rays will reflect in a particular manner. These reflections are known as regular reflections.
Diffuse/irregular reflection
When light rays hit a rough surface, the reflected rays scatter in different directions. However, the law of reflection still applies.
The normal is still perpendicular to the surface, however due to the ‘bumps’ in the rough surface the normal is tilted at an angle.
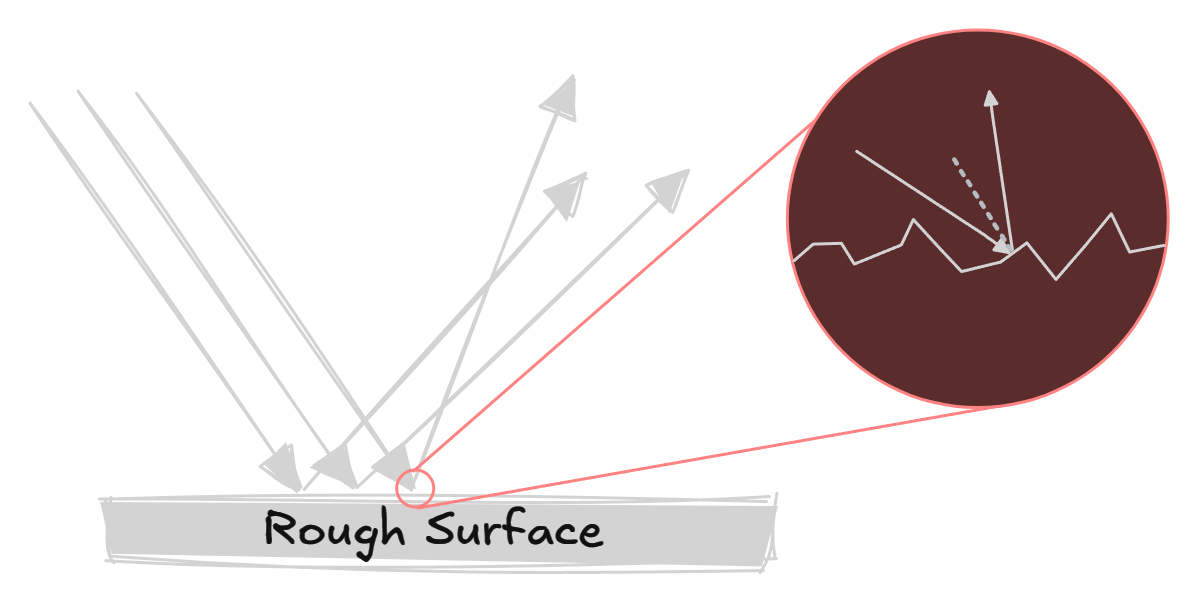
Normal line
A normal line is an imaginary line perpendicular (at a right angle) to the surface.
This line divides the angle between the incident ray and the reflected ray into two equal angles.
Real and virtual images
A real image is formed when light rays converge onto a point, creating a focal point. This is where the image is, and it is ‘actually there,’ your brain is not tricking you.
A virtual image is formed when light rays appear to be diverging (spreading apart) from a single point (like an imaginary focal point) behind the mirror.
Flat/plane mirrors
The properties of the image formed in a flat mirror include:
- An upright image, not upside down.
- The same size as the real object — the mirror does not magnify the image.
- A laterally inverted image (reversed sideways, so if you wave your left hand it will look like you waved your right hand).
- A virtual image — the image appears to be formed behind the mirror, however it has not.
- The distance of the image formed ‘behind the mirror’ is equal to the distance of the object in front of the mirror.
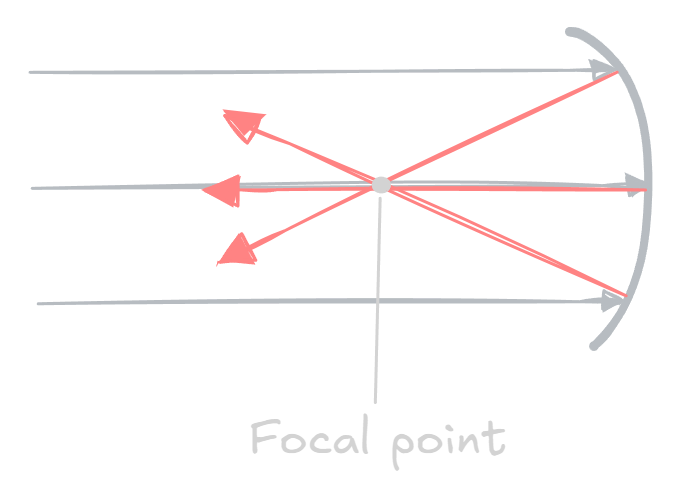
Concave mirrors
A concave mirror is a mirror that is curved inwards. It is called a converging mirror because it causes all the light rays to meet at a single point — the focal point.
When far away, the image produced from a concave mirror is a real image, inverted (the object is upside down) and diminished (smaller than the object). The image can be seen at, or in front of, the focal point.
As the object approaches the mirror, the image becomes enlarged in size, but it is still inverted (upside down).
Eventually once the object gets very close to the mirror and passes the focal point, the image becomes virtual and upright, looking like it is behind the mirror. However, it is still enlarged.
Therefore, concave mirrors can produce both real, inverted images, and also virtual, upright images depending on the distance of the object.
Convex mirrors
A convex mirror is one that curves outwards. It is called a diverging mirror because it causes all the light rays to diverse or spread outwards.
The light rays appear to be coming out from a point behind the mirror — an imaginary focal point.
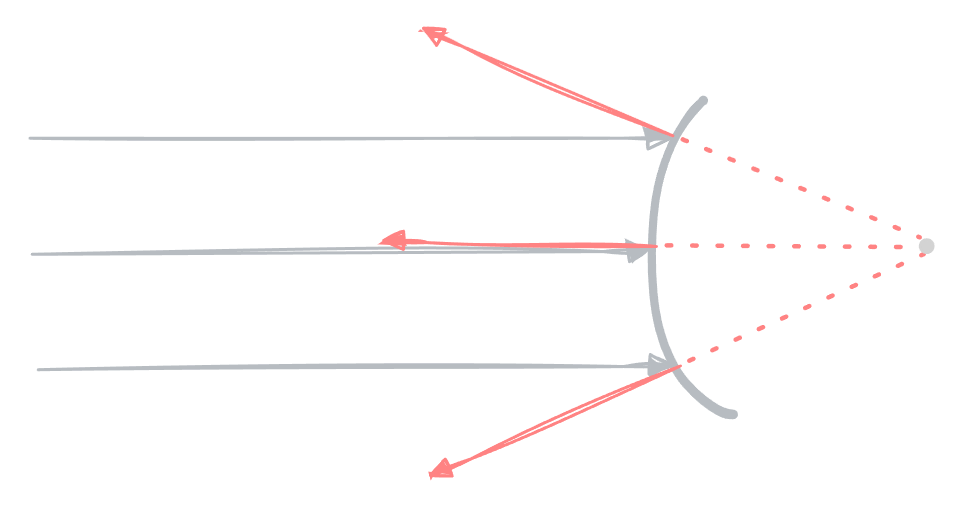
The image produced by a convex mirror is always virtual and appears to be located behind the mirror.
When the object is far away, the image is upright and diminished. As the object approaches the mirror, the image forms closer to the mirror, and grows larger, but it is still diminished.
Therefore, convex mirrors always produce virtual, upright images, that are also diminished.
Refraction
Refraction is the bending of light as is passes from one medium into another.
When light travelling through one material reaches a second material, some of the light will be reflected, and some of the light will enter the second material.
When this happens, it will bend and travel in a different direction than the reflected light. This is called refraction.
Why it occurs
Light travels at different speeds through different mediums. As light passes through from one substance into another, the difference in speed results in different amounts of bending taking place.
The refractive index is a measure of how easily light travels through a substance. The lower the refractive index of a material, light will travel faster through this material, and it will bend less.
If light enters any substance with a higher refractive index, it slows down (such as from air into glass). The light bends towards the normal line, and the angle of refraction is smaller than the angle of incidence.
This is the opposite when light enters a material with a lower refractive index, it speeds up. The light bends away from the normal line, and so the angle of refraction is larger than the angle of incidence.

Lenses
A lens is a transparent piece of plastic or glass that is shaped to curve outwards or inwards. Lenses refract light by either converging or diverging the light rays.
Convex (converging) lenses
A convex lens curves outwards, causing light rays to come together and converge.
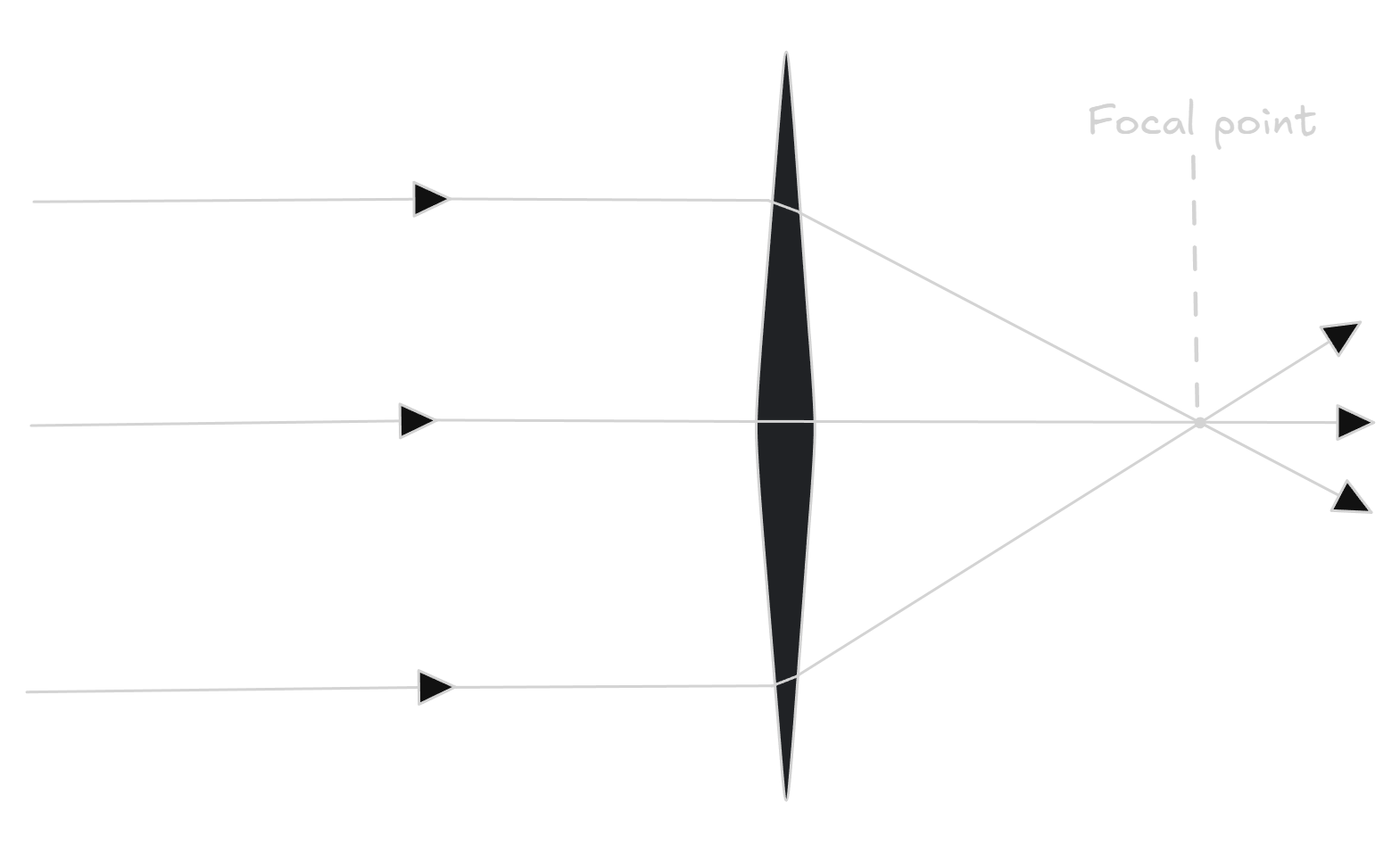
If a convex lens is held close to an object, it can be used as a magnifying glass. In this case, it produces an upright, enlarged and virtual image.
It is virtual because the image is formed by rays that don’t actually meet, but instead they seem to diverge from an imaginary point in front of the lens.
If light reaches a convex lens from a distance, the convex lens can be used to focus the light on a screen to form an image, for example this happens at a cinema. This image also forms by the convex lenses in your eyes.
This image will be real, inverted (upside down) and diminished, and the further away the object is, the smaller the image will be. A real image forms because the light rays actually meet at a point.
Concave (diverging) lens
A lens that curves inwards is called a concave lens. It causes light to diverge (spread out).
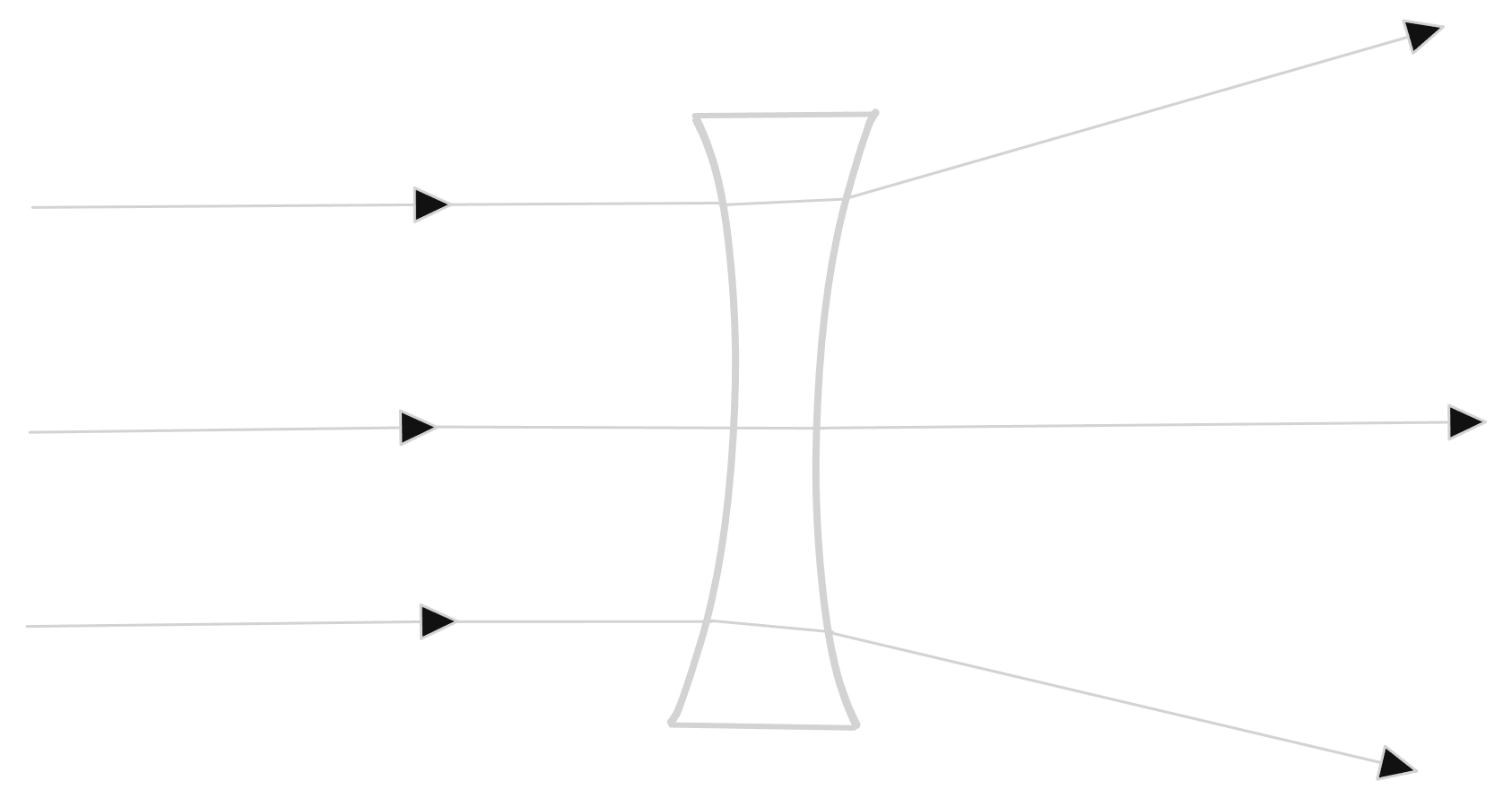
The mirror spreads the light rays as though the rays have come from a single point behind the lens (virtual focal point). It produces a diminished, upright and virtual image.
Depth illusion
When someone is standing in a swimming pool, their legs look shorter than normal. This depth illusion happens because light from an object under water is bent away from the normal when it leaves the water into the air, refracting the light exiting the water.